Any Colour You Like: Pigmentation and Photosynthesis
To understand color, we first have to examine light. What we see as visible light is actually a small portion of the electromagnetic spectrum. This spectrum includes everything from long, low-energy radio waves all the way up to short, high-energy, Hulk-inducing gamma rays (do not try this at home). Technically, all energy on this spectrum is radiation, but most of it doesn’t have enough energy to do any damage to us. The frequencies that can do damage are typically filtered by our atmosphere or blocked by the magnetic field produced by Earth’s liquid outer core. Some ultraviolet rays still penetrate the atmosphere, which is why you should wear (ocean-safe) sunscreen. These rays are made up of photons, or little packets of energy, and the energy of the photon goes with its wavelength. To make this more intuitive, imagine you’re floating on an inner tube in the ocean: long, rolling waves are gentler and calming (low energy), while short, oscillating waves are disruptive (high energy). Between the longest and shortest wavelengths of the electromagnetic spectrum is the visible light we all perceive. The different colors of light we see are just different wavelengths of photons—when light moves through a medium, such as air or glass, the difference in “speed” between wavelengths is seen as color.
Here’s a quick video of Astrophysicist and Cool-Tie Sporter Neil deGrasse Tyson explaining the origin of the spectrum discovery:
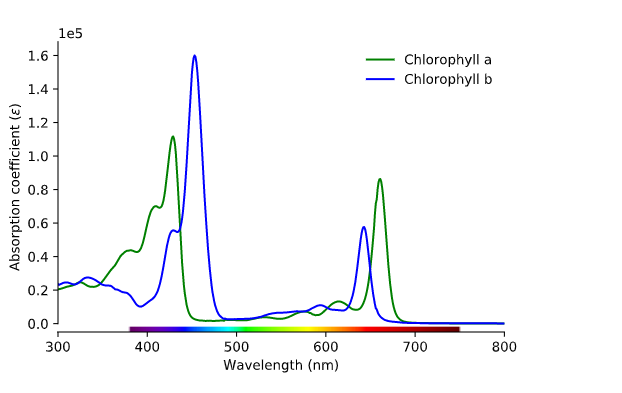
Now we know what light is, but how does this affect the color of plants? Plants produce pigment molecules, or chemical structures, that absorb light to be used in an energy transfer reaction called photosynthesis. Photosynthesis actually predates plants. About 3.5 billion years ago, cyanobacteria, also known as “blue-green algae,” were the first organisms to directly use the power of the sun to produce energy. Today, cyanobacteria, algae, and plants all contain chlorophyll a. Plants also contain chlorophyll b and carotenoids, both of which are known as accessory pigments. Chlorophyll appears green because it absorbs blue and red light. So in reality, plants seem green because they’re not using green light but reflecting it.
Carotenoids, which are less abundant and therefore visually masked by chlorophyll during much of the growing season, reflect longer wavelengths of light—red, yellow, orange. To remember this, just think carrot-enoid. When shorter days foretell the coming frost, deciduous plants dismantle the valuable chlorophyll-housing chloroplasts and absorb the molecules into central tissues for the winter. This reveals the dazzling (reflected) carotenoid colors of fall!
As explained earlier, ultraviolet radiation tends to have too much energy for living beings. To combat this, some plants contain purple pigments, or anthocyanins, which don’t participate in photosynthesis but protect plants from the high-energy photons.
Photos courtesy of Wikimedia Commons and NBGS